Which Elements Don T Follow the Octet Rule
Hydrogen has only one valence electron and only one place to form a bond with another atom. The two elements that most commonly fail to complete an octet are boron and aluminum.

7 3 Lewis Symbols And Structures Chemistry
However many atoms below atomic number 20 often form compounds that do not follow the octet rule.
. Key Takeaways The Octet Rule and Its Exceptions. Hydrogen Fluorine Sulfur Carbon Chlorine Oxygen Question 32 Status. Another exception of octet rule is transition elements.
Which one of the following compounds does not follow the octet rule. Boron usually has six electrons such as with BH3 BOH3 etc sulfur and phosphorous can often make more than four bonds. As the octet rule requires eight electrons around each atom a molecule with an odd number of electrons must disobey the octet rule.
Hydrogen and helium are special cases that do not follow the octet rule but the duplet rule. Likewise people ask which atoms can violate the octet rule. How can atoms join together.
Hydrogen beryllium and boron have too few electrons to form an octet. Select one or more. Transition metal complexes these usually follow.
Molecules through an odd number of electrons are fairly rare in the s and also p blocks but rather common. The two elements that most commonly fail to complete an octet are boron and aluminum. Octet rule is more advisory than a rule.
View the full answer. Chlorine atom in the compound has ten electrons in its outer shell so it doesnt follow the octet rule. Rather they both follow a special rule sometimes called the duet rule.
As you go down the periodic table orbitals get closer in e. Free Radicals Some elements most notably nitrogen can form compounds that do not obey the octet rule. Chlorine and sulfur will not strictly follow the octet rule.
The molecule has an odd number of valence electrons. There are also a. Elements like hydrogen lithium helium do not obey the octet rule.
Although they are few some stable compounds have an odd number of. Due to the presence of d-orbitalsthey can hold 18 electrons in its outermost shell. Not yet answered 1 Points possible.
Molecules with unpaired electrons are termed free radicals. The octet rule states that atoms below atomic number 20 tend to combine so that they. 100 In the Water of Hydration lab what is the.
Some elements that disobey the octet rule include. The central atom is from Group 2A or 3A. Beryllium has only two valence atoms and can form only electron pair bonds in two locations.
They contain an s s s orbital but no p p p orbitals. Dodecaborane with 6-coordianted boron. Boron has three valence electrons.
There is another rule called the duplet rule that states that some elements can be stable with two electrons in their shell. Explaination Expanded octet can be seen in atoms that have vacant orbital this is an essential requireme. Depending on counting system boron clusters fe.
On the other hand some elements exhibit hypervalency and have the ability to form hypervalent molecules. The central atom is surrounded by more than four atoms or eight valence electrons. Which elements do not strictly follow the octet rule when they appear in the Lewis structure of a molecule.
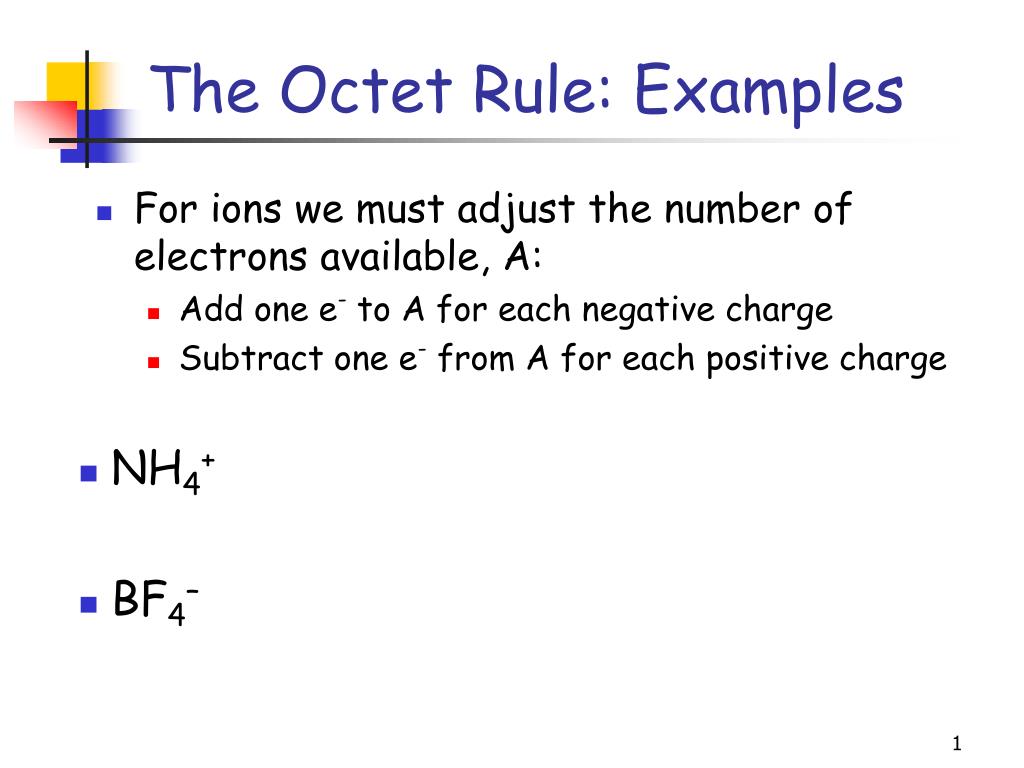
Boron and Aluminum follow the Sextet Rule. A NF3 b CF4 c SF4 d PH3 e HCl I know I can eliminate A and B because nitrogen and carbon follow the octet rule. Which of these do not obey the octet rule.
In most cases the two elements that fail to complete an octet are boron and aluminum since they both readily form compounds with six valence electrons rather than the usual eight predicted by the octet rule. BCl3 do not obey octet ruleIt is a electron deficient moleculeAs it share only three electron with chlorine atom. List Four Elements that do not Obey the Octet Rule.
I know I can eliminate E because the total. Odd-electron molecules represent the first violation to the octet rule. Nitrogen molecule will be achieving octet by sharing three electrons.
The octet rule states that for atoms to be stable they must have eight electrons on their outermost shells. Elements that do not follow the octet rule. This is the best answer based on feedback and ratings.
Select one or more. The Lewis structure of a molecule has a high probability of violating the octet rule if 1. Another exception of the octet rule is transition elements.
Save for oxygen nitrogen carbon and fluorine most other elements dont follow the octet rule. First for elements in periodic system He-configuration 3-valent boron compounds. 100 Which elements do not strictly follow the octet rule when they appear in the Lewis structure of a molecule.
The two elements that most commonly fail to complete an octet are boron and aluminium. General exception to the octet preeminence include molecule that have an odd number of electrons and molecules in i m sorry one or an ext atoms possess much more or fewer 보다 eight electrons. They both readily form compounds in which they have six valence electrons rather than the usual eight predicted by the octet rule.
Hydrogen H and helium He does not follow the octet rule when forming bonds with other elements. Its usually obeyed by the main group elements. Elements like hydrogenlithiumhelium do not obey the octet rule.
Atoms come together to form molecules because of their electrons. Whereas fluorine molecule will be achieving octet by sharing one electron. Answer 1 of 5.
Exception are paramagnetic compounds obvious reason. Since 1p subshells do not exist some elements find stability in 1s 2 configurations. They both readily form compounds in which they have six valence electrons rather than the usual eight predicted by the octet rule.
Not yet answered I Points possible. They both readily form compounds in which they have six valence electrons rather than the usual eight predicted by the octet rule. They can only lose or gain one electron to become stable due to which they follow the octet rule.
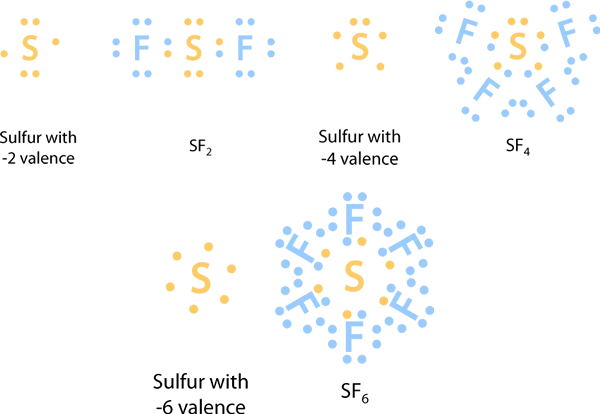
Exceptions Of The Octet Rule Study Guide Inspirit

Solved Which Of The Following Molecules Does Not Obey The Octet Rule A Mathrm Hcn B Mathrm Pf 3 C Mathrm Cs 2 D Mathrm No E None Of These
Ppt The Octet Rule Examples Powerpoint Presentation Free Download Id 312520
Chapter 8 Basic Concepts Of Chemical Bonding Jennie

Exceptions To The Octet Rule Chemistry Master

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
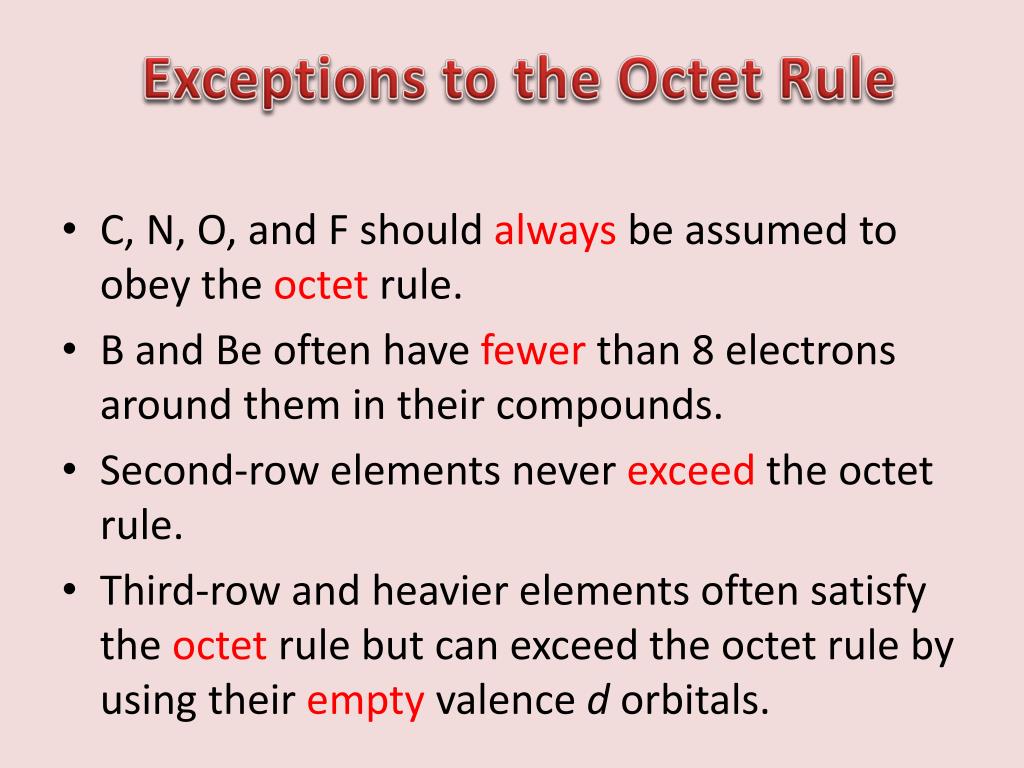
Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 5318726
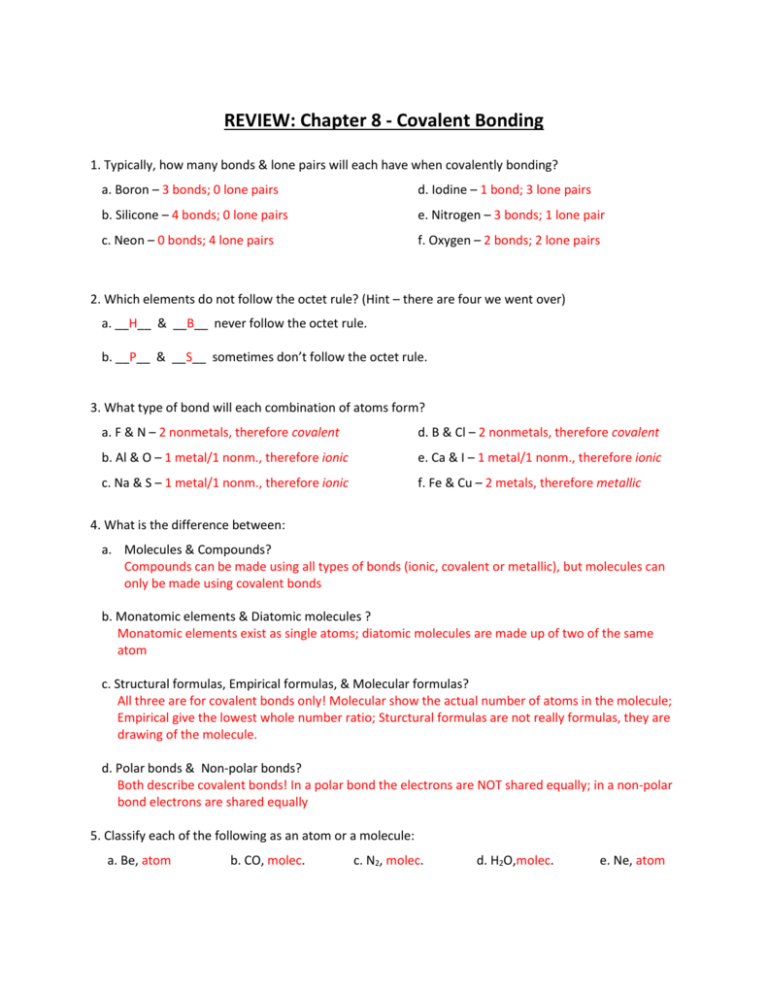
Review Chapter 8 Covalent Bonding
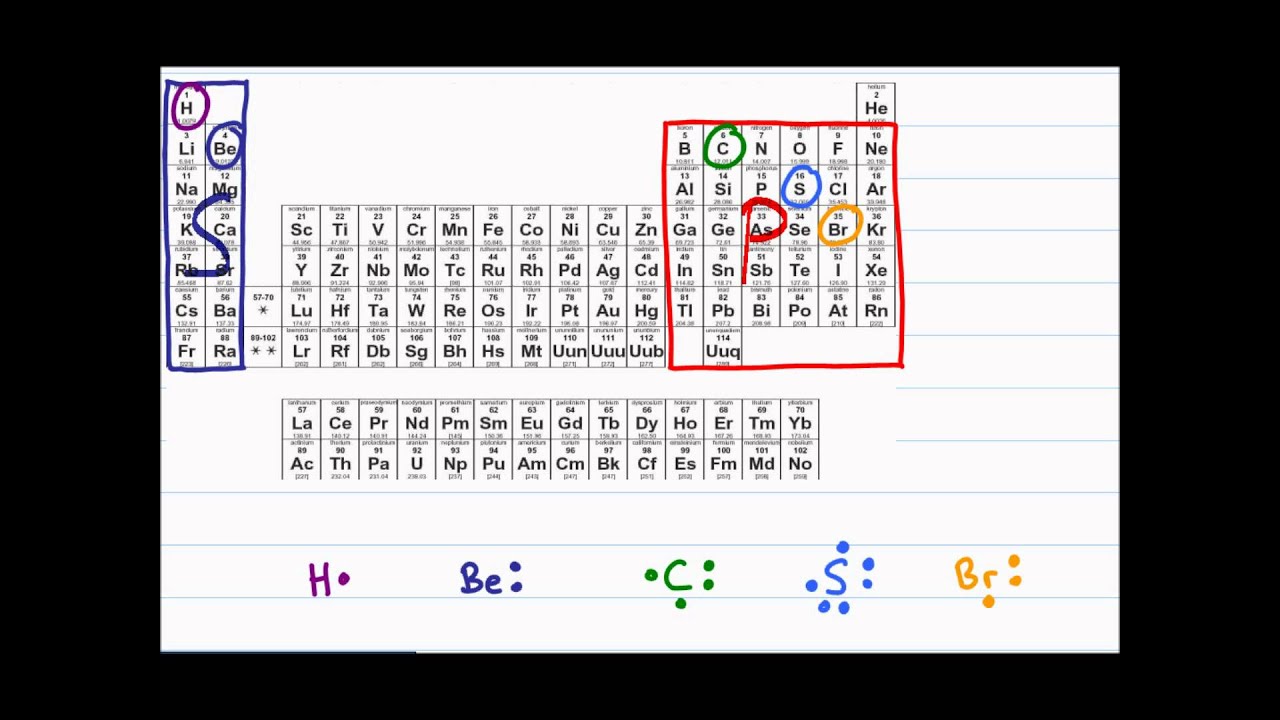
Lewis Dot Diagram And Octet Rule Youtube
8 1 Lewis Symbols And The Octet Rule Chemistry Libretexts

Exceptions To The Octet Rule Youtube

Solved 3 For Which Of The Following Lewis Dot Structures Does The Atom X Follow The Octet Rule Select All That Apply 4 Based On The Information In Your Lab Manual Which Of
Chapter 8 Basic Concepts Of Chemical Bonding Ppt Download
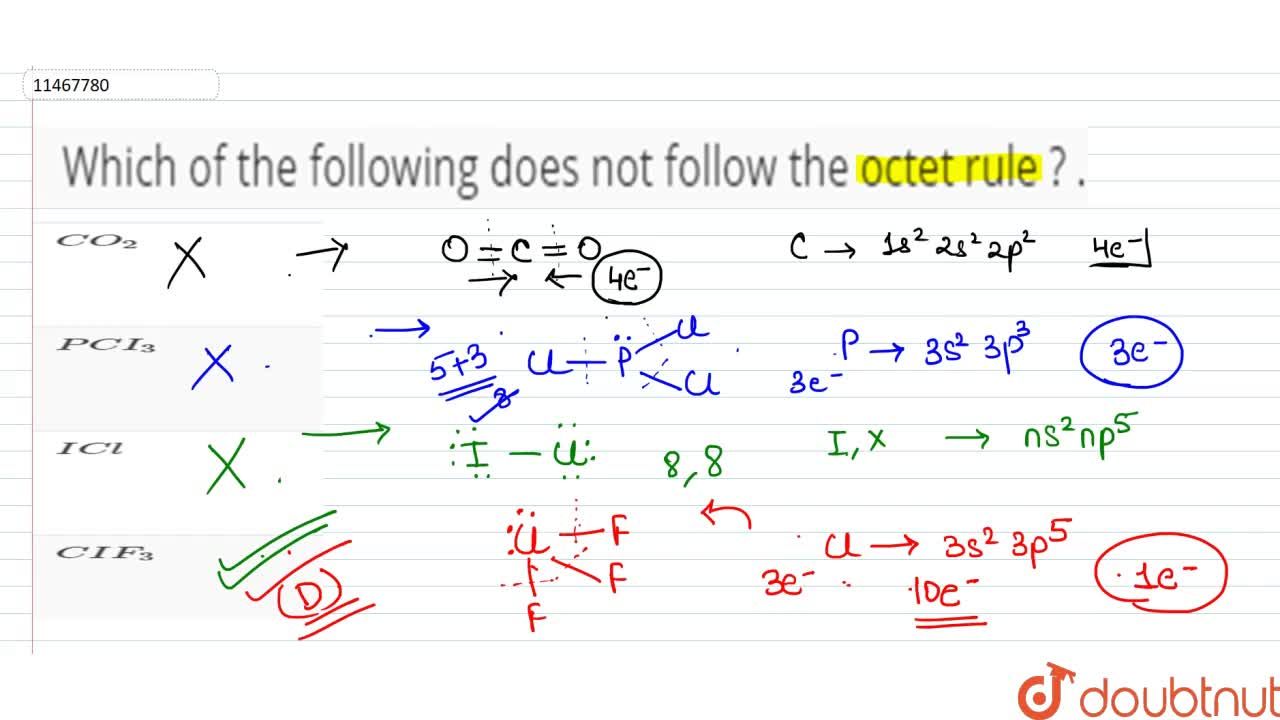
Which Of The Following Does Not Follow The Octet Rule

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube

Exceptions To The Octet Rule Video Khan Academy

Ppt The Octet Rule Powerpoint Presentation Free Download Id 5075250
Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 3528141

Comments
Post a Comment